| |
 |
|
|
 |
| |
 |
|
|
::: NTU Achieves Major Breakthrough in Molecular Biomedicine: Professor Zee-Fen Chang Unfolds the Moving and Dying Mechanism of Chronic Myeloid Leukocytes
|
After years of strenuous efforts, Professor Zee-fen Chang from NTU's Institute of Biochemistry and Molecular Biology finally unfolds the moving and dying mechanism of chronic bone marrow leukocytes, providing a slim chance of survival for malignant chronic myeloid leukemia patients who were threatened by resistance to certain drugs. Professor Chang's research paper has been accepted and published in the prestigious "Journal of Molecular Cell Biology."
According to Dr. Chang's research, chronic myeloid leukemia results from a malignant tumor produced by the translocation of the 9th pair and the 22th pair of chromosomes, and the characteristics of which are marked by abnormal hper-plasmia of leukocytes and splenomegaly. Every year Taiwan has about two hundred new patients suffering from CML. Recent studies have shown that, after the translocation of these two pairs of chromosomes, a carcinogenetic gene product called BCR-ABL is formed, which, other than being anti-apostosis, stops the white blood corpuscles from splitting up. Even treated with an exclusive BCR-ABL inhibitor called Gleevec, CML patients' life expectancy can be increased by as many as fifteen years, studies have shown that about 20 percent of CML patients develop resistance to Gleevec, hence their lives are constantly in danger.
|
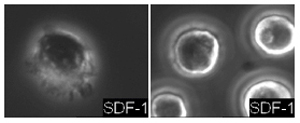
Stimulation by SDF-1. On the left are normal cells, on the right are Bcr-Abl transformation cells.
Bcr-Abl transformation cell lines. On the left are the ones without drug treatment, on the right shows apostosis after treatment with Gleevec.
|
|
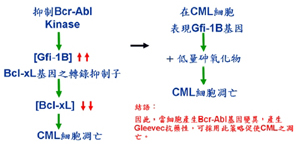
Dr. Chang indicated that, in the process of using Gleevec to inhibit BCR-ABL, a gene called GFi-1B increases its activity, which can disband the anti-apostasis function of CBC-ABL, and reduce the resistance of CML patients to Gleevec, thus improving the result of treatment.
According to this research finding, if researchers could find a chemical compound that could increase the activities of the Gfi-1B genes, they could then further disband the anti-apostosis function of BCR-ABL, and strengthen the poison-kill effect of low dosage arsenic oxides on CML, thus allowing the patients to live longer by lowering their resistance to Gleevec. This method falls in line with the "cocktail therapy" concept, which espouses the idea of "combating poison with poison." As a result, this treatment method could constitute a new therapy for CML
* Dr. Zee-Fen Chang's Personal Website:
http://www.mc.ntu.edu.tw/department/ibmb/chinese/teacher/ChangZeeFen/ChangZF_set.htm
|
Copyright 2008 NTU Secretariat
|
|
Copyright © 2006 National Taiwan University
No. 1, Sec. 4, Roosevelt Road, Taipei, 10617 Taiwan(R.O.C.)
Phone: +886-2-3366-3366 Fax: +886-2-2362-7651
|